Powerful, Easy-to-Use, Lightning-Fast
Quality Management Software (QMS) for Manufacturing
1factory provides QMS software for manufacturers operating in a wide range of industries from aerospace to medical devices, automotive, industrial, and semiconductor industries.
Author, approve, and version-control documents. Automate training assignments and track completion. Manage NCRs, CAPAs, and complaints.
Go live in days, ace audits effortlessly, and maintain complete regulatory compliance for ISO 9001, AS9100, ISO 13485, 21CFR Part 11 and more.
AUTOMATE COMPLIANCE. ACE YOUR AUDITS.
80% REDUCTION IN AUDIT PREP TIME
95% of the team was onboarded in 60 days. The new digital QMS reduced audit preparation time by 83%. Document retrieval time plummeted from 15 minutes of searching to under 1 minute, unlocking time savings across every search query.
"It takes all the complexity of compliance, documentation, and process control and makes it as straightforward as flipping a switch."
Tony Sonson, Director of Operations, Vidar an ITT Company
ACE YOUR AUDITS
"Both our AS9100 and ISO13485 auditors were extremely impressed with 1Factory's ease of use. I would 100% recommend the transition to 1Factory's Quality Management Software."
"It has helped Fairview go completely paperless over the past 3 years while helping us maintain exceptional traceability across the board"
Harrison Moulton, Quality Manager, Fairview Machine Company
OVERVIEW
WHAT IS A QUALITY MANAGEMENT SYSTEM (QMS)?
A Quality Management System (QMS) formalizes the business processes and procedures used to ensure that manufactured products consistently meet customer, industry, and regulatory requirements. For manufacturers in sectors such as aerospace, medical devices, automotive, semiconductor, industrial equipment, and precision machining, a QMS provides the structured framework necessary to coordinate interconnected workflows across the entire organization.
This system addresses the fundamental challenge that quality defects typically originate from process failures, such as poorly designed processes, incorrect execution, or failed handoffs between steps. A formal QMS manages these risks by version-controlling hundreds of interlinked standards and procedures to ensure every team member follows the latest approved revision. It further ensures workforce competency through automated training tracking and maintains searchable records for every business process - from product development and manufacturing to issue resolution - to provide definitive evidence for auditors.
Without a digital system, manufacturers often face fragmented paper-based silos, slow document retrieval, and burdensome audit preparation that consumes days of engineering and quality staff time.
THE 1FACTORY QUALITY MANAGEMENT SOLUTION
The 1factory platform provides a unified, digital environment that simplifies every aspect of quality management while drastically reducing compliance risk. To solve document control and handoff challenges, the system offers a centralized hub with automated lifecycle management for authoring, redlining, approvals, and electronic signatures to ensure only the latest, approved processes are in use.
The platform eliminates competency-related gaps by automating training workflows that assign and track training by role for new hires, role changes, or revised procedures, ensuring the entire workforce is qualified to execute their specific process steps. By maintaining searchable records across all business processes - from product development and manufacturing to issue resolution - 1factory provides a single source of truth that accelerates record retrieval to under a minute and reduces audit preparation time by over 80%.
1factory serves a wide range of manufacturers, from precision machine shops to global enterprises, by combining extraordinary ease of use and rapid migration with an audit-ready architecture that maintains rigorous ITAR and CMMC compliance on AWS GovCloud.
FEATURES: QUALITY MANAGEMENT SOFTWARE
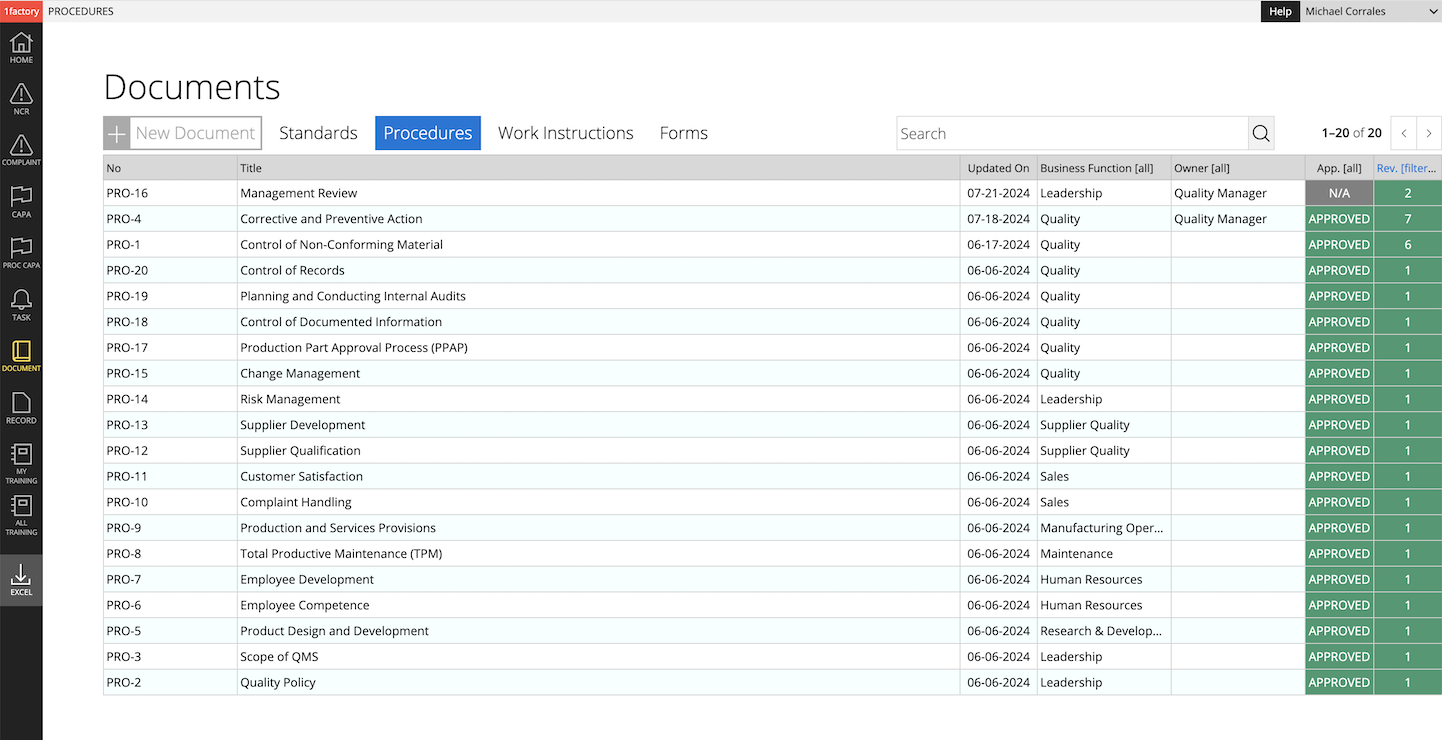
1 Standards & Requirements
Manage all external standards (e.g. ISO 9001, AS9100, ISO 13485, TS16949), customer-specific requirements (e.g. supplier quality manual), and regulatory requirements (e.g. 21 CFR Part 820) in a single centralized quality management system. Digitize requirements and make them easy to find with 1factory's document control capabilities.
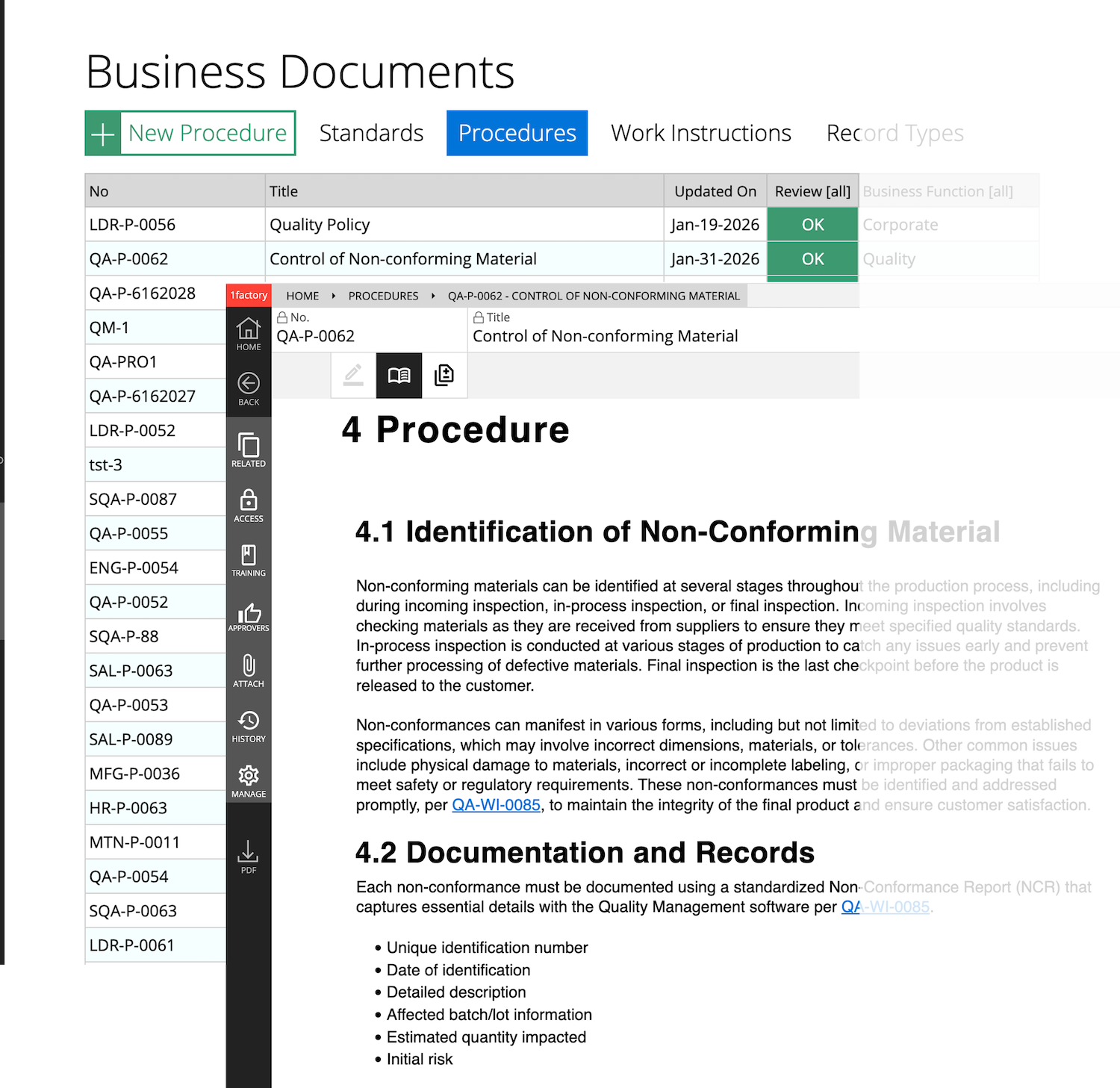
2 Procedures & Work Instructions
Author procedures and work-instructions with text and images using our document authoring features. Enjoy powerful version control, with clear change history across revisions. Establish clear links between standards (requirements), procedures, and other related documents with 1factory's digital document control system.
3 Forms & Records
Manage Forms (e.g. Document Change Order) with revision control and approvals within the 1factory QMS system. Ensure that records are generated only with the latest version of a form. Route records for approval. Easily search and retrieve records for audits.
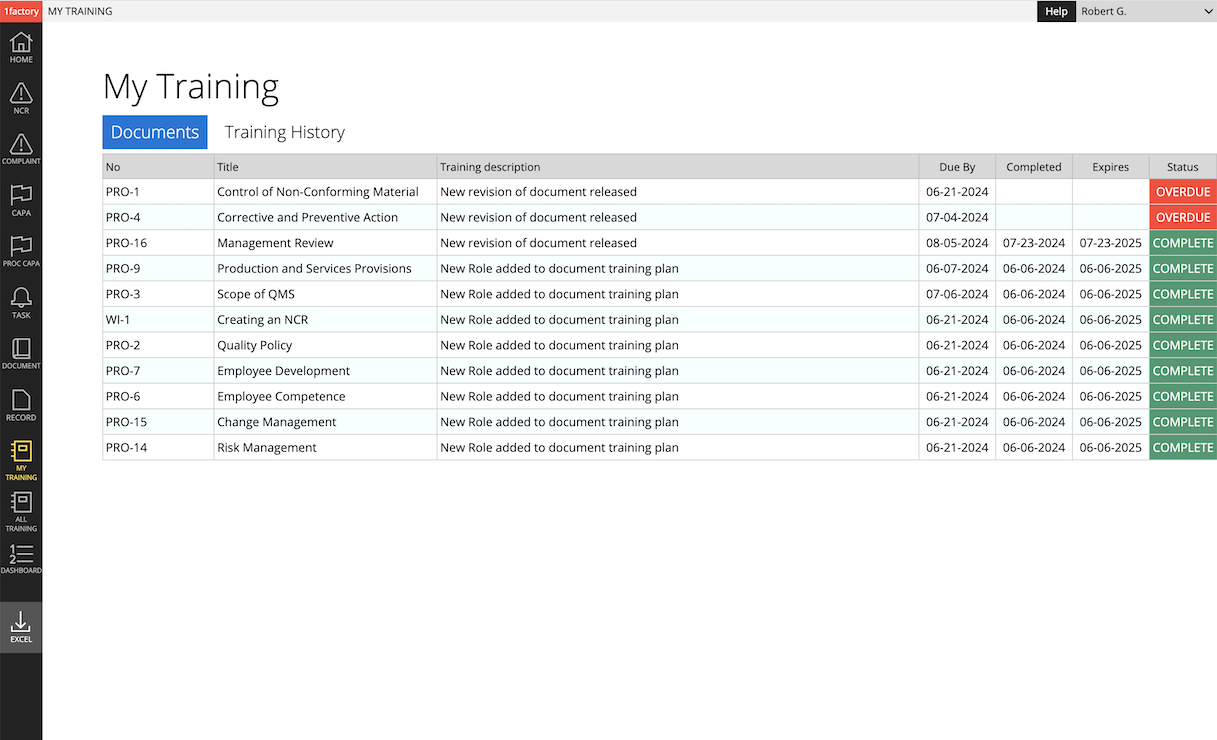
4 Training Management
Simplify training management. Establish training plans. Easily assign and track training by role and user. Generate training for (1) initial release & new revision of a procedure, (2) new hire or role change, (3) scheduled periodic retraining, and (4) retraining as part of corrective action. Ensure training is always up-to-date and avoid audit findings with 1factory's powerful QMS Training Management capabilities.
5 Multi-Site Deployments
Effortlessly manage corporate and site-specific procedures and work instructions with 1factory's Federated (Multi-Org) QMS capabilities. Corporate documents apply to all or selected sites, while site-specific documents apply to only the specific site. Effortlessly assign and track approvals and training across all sites using 1factory's powerful Federated Quality Management Software.
6 Access Controls
Protect critical documents and organizational intellectual property - procedures, work instructions, and records - by limiting access to specific user roles with 1factory's powerful QMS access control features.
7 Approval Workflows
Route documents - procedures, work instructions, and records - for approval by role and by user using the built-in workflow engine. Notify users of required approvals and document status changes via email. Record approvals and electronic signatures for traceability and Part 11 compliance.
8 Document Change History & Version Control
Easily manage changes with powerful built-in document change history, redline view, and version control capabilities integrated into our quality management platform. Simplify document review and approval with the powerful redline view that highlights additions, deletions and edits.
9 Document Effectivity
Easily set document effectivity by date, or by training period (e.g. document is automatically effective at the end of the 15 day training period), or by percentage trained (e.g. document is effective after 95% of employees are trained). Effortlessly ensure only effective documents are available for end-user use.
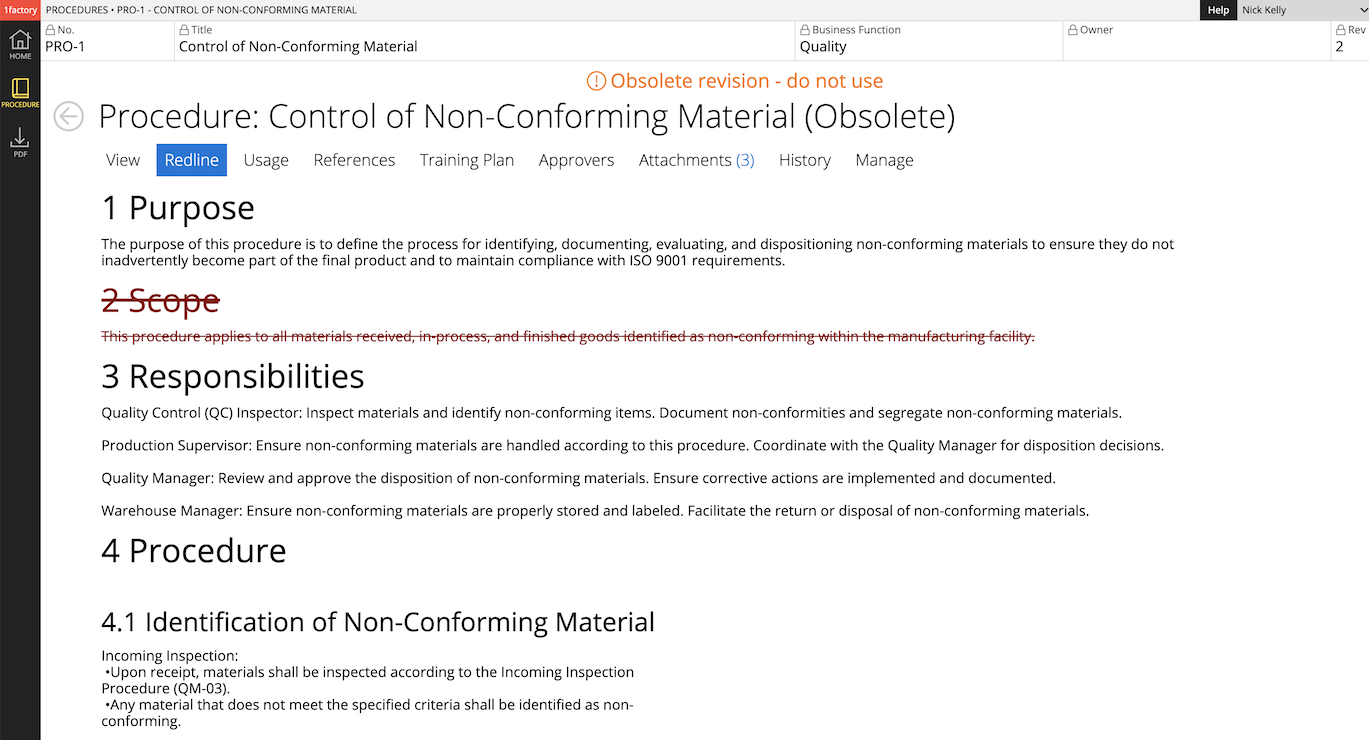
10 Nonconformances (NCRs)
Easily create and manage nonconformances for manufacturing or engineering caused defects, deviations, customer returns, audit findings, safety problems etc. using our QMS Software. Auto-populate NCR details from Inspections. Assign action-items, and use automated reminders to ensure timely resolution.
11 Corrective Actions (CAPAs)
Initiate CAPAs and SCARs from NCRs using our quality management software. Setup CAPA templates to guide the user, and ensure a consistent problem-solving process. Track CAPA and SCAR progress, and use automatic reminders to ensure timely resolution. Review and approve through our quality management system.
12 Supplier Corrective Actions (SCARs)
Request Supplier Corrective Actions from suppliers using the 8D process through the 1factory Supplier Portal. Work together with suppliers to resolve problems and prevent recurrence. Ensure all 8D sections are filled-out, supplier actions are completed and effectiveness is verified prior to closing out the corrective action.
13 Complaints
Record and track product failures or deficiencies reported by field service teams and customers using our quality management system. Track reported issues from initial complaint through Investigation (Review of Device /Lot History Record, Failure Analysis), Risk Assessment, Containment, Corrective Action, and Preventive Action. Assign and track actions within the QMS Software platform.
14 Audit Findings
Manage audit findings and associated tasks with 1factory's Quality Management Software. Support a continuous improvement culture by enabling team-members to log and track business process improvement actions. Ensure compliance and readiness for customer or regulatory audits.
15 Quality Matrix / Dashboard
Get a birds-eye view of your QMS and audit-readiness with the Quality Management Dashboard. View a hierarchical view of Standards, Requirements and associated Procedures. Simplify audit-prep by ensuring all documents up-to-date and all required training is complete.
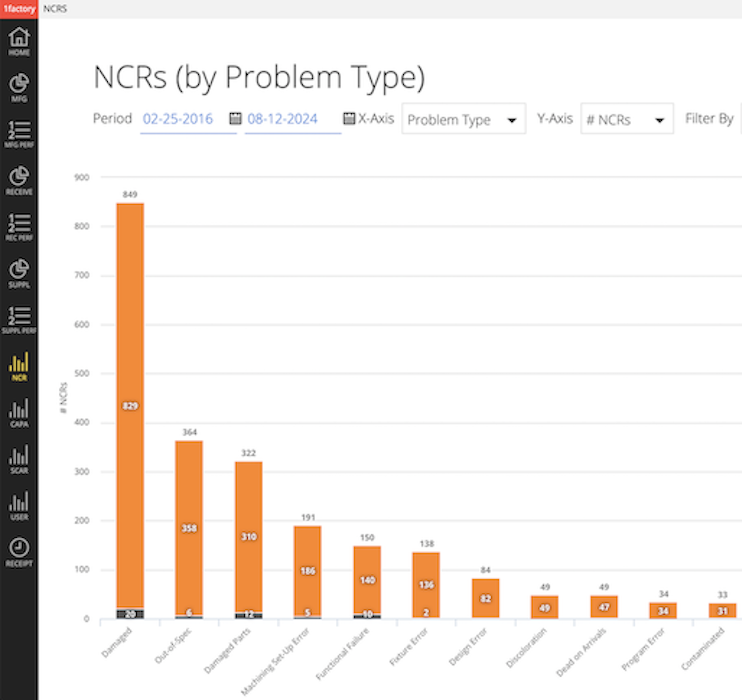
16 QMS Analytics & Reporting
Easily pareto nonconformances, CAPAs, SCARs and Complaints by part number, type, supplier, detected at, caused by and other fields using our QMS Software analytics. Deliver CAPAs and Suppliers electronically within 1factory to connected Customers and Suppliers or email CAPAs and SCARs to customers and suppliers.
17 QMS Deployment and Go-Live
1factory has the fastest QMS Software deployment time on the market. With 1factory's powerful onboarding tools, you can go-live within a week. In comparison, other Quality Management Software systems will take months or even years to deploy.
CASE STUDIES
VIDAR (an ITT Company)
VIDAR (an ITT Company) manufactures advanced industrial motors with embedded variable-speed intelligence for critical sectors like chemical processing, water, and energy.
VIDAR faced challenges with a fragmented, paper-based Quality Management System (QMS) that resulted in slow production and burdensome audit preparation.
The new digital QMS reduced audit preparation time by 83% (from 3 days to 4 hours). Document retrieval time plummeted from 15 minutes of searching to under 1 minute, unlocking time savings across every search query. Supplier Corrective Action Request (SCAR) closure time dropped by 70%, positioning VIDAR for ISO 9001 certification.
Tony Sonson, Director of Operations: "It takes all the complexity of compliance, documentation, and process control and makes it as straightforward as flipping a switch."


Fairview Machine - Aerospace, Medical Devices
Fairview Machine is a manufacturer or high precision Aerospace and Medical components. Fairview Machine recently deployed the 1factory quality control system and digitized their quality management processes.
Harrison Moulton, Quality Manager at Fairview Machine Company, says:
"I would 100% recommend the transition to 1Factory's Quality Management Software. It has helped Fairview go completely paperless over the past 3 years while helping us maintain exceptional traceability across the board."
Read the full case-study to learn how Fairview Machine transitioned the entire organization from a paper-based quality system to a paperless quality management solution.
EASY TO USE. LIGHTNING FAST. SECURE & RELIABLE
INTUITIVE DESIGN & LIGHTNING FAST SPEED
1Factory's intuitive interface and streamlined workflows make training effortless - most teams achieve full deployment in under two hours with zero operational disruption.
Speed is non-negotiable on the shop floor. Every interaction - from data entry to ballooning to search - happens instantaneously. Your manufacturing operations run at full pace, never waiting on software. With 24/7/365 global availability and real-time analytics, 1Factory keeps your quality operations moving at the speed of production.
SECURITY & RELIABILITY
1Factory meets the security standards that matter to regulated manufacturers: ITAR-compliant hosting on AWS GovCloud, full NIST 800-171 compliance, and SOC 2 Type 2 certification. Your quality data stays protected and audit-ready.
Over the past decade, 1Factory has maintained >99.99% uptime - because we understand that your quality system can't afford downtime. Data encryption, daily database snapshots, and redundant file storage ensure your critical quality records are always protected and recoverable.
FREQUENTLY ASKED QUESTIONS (FAQ)
What industry standards and regulations does the 1factory QMS help manufacturers comply with?
1factory's Quality Management solution supports compliance with key industry standards including ISO 9001, AS9100 (aerospace), ISO 13485 (medical devices), TS16949 (automotive), and many more. We also address regulatory requirements, and offer a separate environment for manufacturers with software validation requirements (21 CFR Part 820 compliance).
With 1factory, you can centrally manage all standards, regulatory requirements, and customer requirements, link them to your procedures and work instructions, and track training, approvals, and effectiveness from one place. Every change is version-controlled with redlines, effectivity dates, access controls, and routed approvals; electronic signatures are captured for traceability and Part 11 needs. The result is a continuously audit-ready system with full traceability to see who changed what, when, and why.
Does the 1factory QMS support electronic signatures and audit trails for 21 CFR Part 11 compliance?
Yes. 1factory's QMS software includes built-in e-signatures and complete audit trails designed for 21 CFR Part 11 compliance. When e-signatures are enabled, each signing event captures the signer's name, digital signature, date/time, and the meaning of the signature (for example, releasing an inspection plan or accepting a lot).
Signatures are executed with a unique username and password, and the system maintains a durable audit trail to safeguard integrity and traceability, capabilities that help regulated manufacturers satisfy electronic records and signature requirements.
Records and forms follow the same controls, making it easy to route for approval and retrieve evidence during audits. Training assignments and completions are also tracked automatically, further tightening your compliance posture for multi-site deployments.
Does the 1factory Quality Management System support Supplier Quality Management (SCARs, scorecards, audits etc.)?
Yes. 1factory offers a full supplier quality module so you can collaborate with suppliers to identify and resolve issues early, and ensure the quality of parts before they leave your suppliers' factories.
1factory's supplier quality management module delivers automated supplier scorecards and dashboards fed by real-time supplier inspection data (measurements recorded at the supplier) and incoming-quality data (recorded at receiving inspection).
Does 1factory support Advanced Product Quality Planning (APQP), PPAP, and FAI processes?
Yes. 1factory's manufacturing quality control module provides built-in capabilities to support the New Product Introduction process, including FAI, PPAP, Control Plans, PFMEAs, Process Flows, Gage R&Rs, Measurement Data, Process Capability Analysis and more.
What industries does 1factory support? Can the QMS support multi-site or global operations with shared documents?
The 1factory QMS supports manufacturers across a wide range of industries such as Aerospace, Defence, Medical Devices, Industrial Equipment, Injection Molding, Machining, Sheet Metal Fabrication, Flow Control, Gear Manufacturing, Firearm Manufacturing and many more.
1factory is the only QMS product on the market with powerful Federated (Multi-Org) QMS capabilities which allow manufacturers to effortlessly manage corporate-wide and site-specific documents (procedures and work-instructions), as well as documents that may be shared across some or all sites.
How long does it take to deploy and go live with 1factory's QMS?
1factory offers the fastest QMS Software deployment time on the market. With our powerful onboarding and data import tools, you can go-live within weeks, compared to months or years with other systems.
How is the 1factory QMS system priced? Do you offer a 'read-only' license type for authors, collaborators, or part-time users?
The 1factory QMS is priced on a simple per-named-user basis (regardless of role), allowing you to assign seats and roles to best support your business.
While other software providers may offer a concurrent-user pricing model, or a tiered-pricing model with different pricing for document authors vs read-only users, at 1factory, we believe in keeping things simple.
With 1factory's simple pricing model, you can easily move people between roles with no impact to overall cost, and enjoy pricing that is 60% to 80% lower than our competitors' pricing.
Please see detailed pricing here.
Are implementation, system validation, and support included in the licensing cost?
Yes. Our per-user subscription pricing includes onboarding and training, data backups, product enhancements, validation test cases, and ongoing support for our QMS software.
There are NO hidden costs with 1factory.
500+ CUSTOMERS. 30+ COUNTRIES. 20+ INDUSTRIES.

AEROSPACE & INDUSTRIAL

AEROSPACE MACHINING

PRECISION MACHINING

MEDICAL DEVICES

AUTONOMOUS AIRCRAFT

ADVANCED CERAMICS