
Powerful, Easy-to-Use, Lightning-Fast
Manufacturing Quality Control Software
Speed-up every step of the QC process from ballooning and inspection planning, to FAI, inspection data collection, and reporting. Automate CMM and Vision System data collection. Monitor and adjust processes with real-time SPC.
Save thousands of engineering hours each year, dramatically improve yield, reduce scrap and rework, and deliver world-class quality.
CUSTOMER TESTIMONIALS
SPEED-UP QUALITY CONTROL TASKS
“1factory is a complete game changer for our quality department. It reduces time spent preparing inspection reports by roughly 66%. Implementation and full integration took 2 hours. If you are not running 1factory, your quality department is behind the times.”
K. Krueger, General Manager, West Valley Precision
IMPROVE YIELD. SHIP WORLD CLASS QUALITY
“With 1factory, problems are identified at the machine, and corrections are made early in the manufacturing process. Our customers have been impressed seeing their parts being inspected at the source of manufacturing with real time data.”
S. Harney, Quality Manager, JWP Manufacturing
OVERVIEW
WHAT IS MANUFACTURING QUALITY CONTROL?
Manufacturing Quality Control (QC) is the process of verifying that products meet engineering specifications through inspection and measurement during production.
QC activities are divided into two primary phases: New Product Introduction (NPI) and Ongoing Production.
During NPI, core activities include drawing ballooning, inspection planning, First Article Inspections (FAI), and the Production Part Approval Process (PPAP) to validate that the manufacturing process is capable of producing parts to requirement.
During Ongoing Production, QC involves the continuous collection of measurement data and the application of Statistical Process Control (SPC) to monitor process stability. By verifying parts at the point of manufacture, QC identify process drift and prevents non-conformances before products are shipped to customers.
THE 1FACTORY MANUFACTURING QC SOLUTION
The 1factory platform provides a centralized environment that simplifies every aspect of quality control across both NPI and Ongoing Production.
For NPI, the software automates drawing ballooning, inspection planning, and FAI and PPAP creation. It replaces manual PPAP preparation with a reusable library of failure modes and control plan elements, cutting PPAP package creation from days and weeks to hours while ensuring compliance with AIAG/APQP and AS13100 standards.
For Ongoing Production, it facilitates direct data acquisition from CMMs, Vision Systems, and digital gages, eliminating manual data entry while performing real-time calculations for process capability and SPC.
With 1factory, manufacturers deliver world-class quality, grow customer confidence and grow their business.
FEATURES
1Drawing Auto-Ballooning
Auto-balloon or point-and-click to balloon drawings in minutes with 1factory's drawing ballooning capabilities. Capture and extract Characteristic, Nominals, Tolerances, GD&T, Fit Tolerances, Threads, Notes, Sheet Number, & Zone to a Plan with just a few clicks. Learn More
2Drawing Rev Comparison
Enjoy fully-automated drawing revision comparison and instantly identify additions, deletions and changes in characteristics, nominals, tolerances, and notes.
3Inspection Plans
Speed-up Manufacturing Control Plan creation. Define Characteristics, Specifications, Inspection Methods, Operation Numbers, and Sampling Rules. Track changes with Plan History and Version Control. Ensure factory-floor is always working to the latest released version of documents.
4Tabulated Plans
Easily manage tabulated drawings with thousands of configurations via a single Inspection Plan for the product family. 1factory's quality control software automatically generates the inspection sheet for each configuration with all required common and part-specific features.
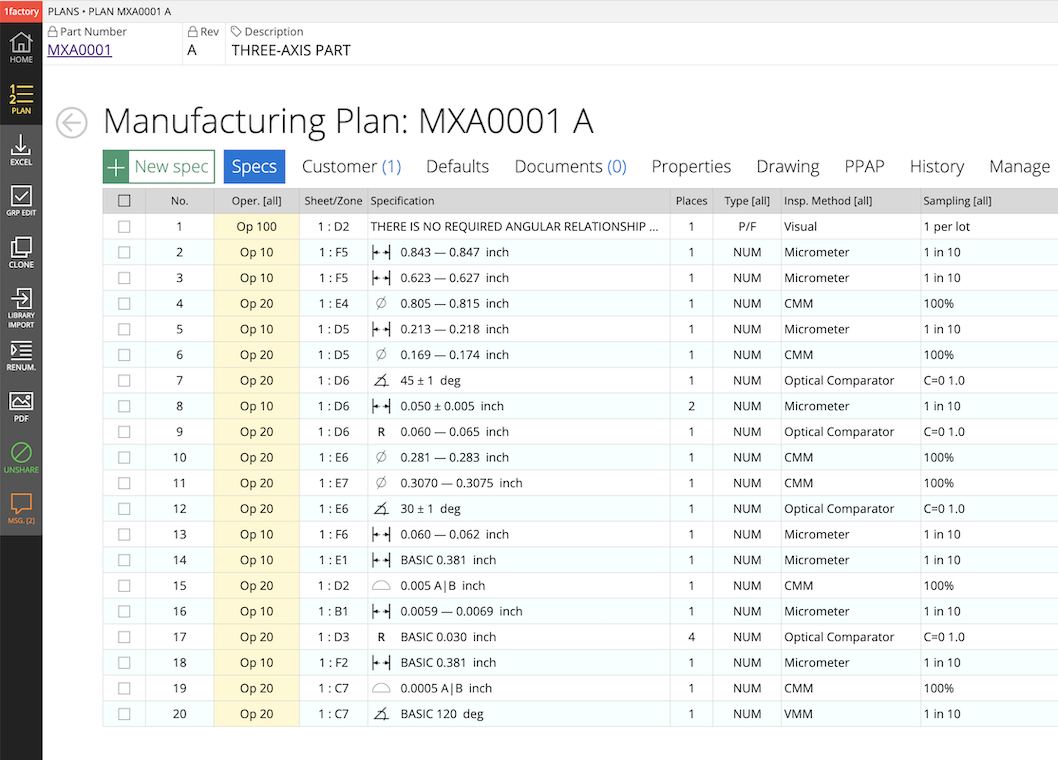
5Spec Library Plans
Create reusable "Libraries of Specs" for industry standards (e.g. ports for hydraulics) and for common inspection elements. Quickly assemble product-specific plans from libraries.
6Unit Conversion
Enjoy automated unit conversion from mm to inch and from inch to mm. So if your customer drawing is in mm, unit conversion activated in the Plan, your factory can record data in inches, and output a report in mm for your customer.
7First Article (FAI, AS9102)
Balloon drawings, import CMM data, and create a First Article Inspection report in minutes with 1factory's powerful Inspection Management Software. Link component FAIs to parent assembly FAI. Output FAIs for easy upload to Net Inspect or other portals.
8Automated CMM Data Import
Automate data collection from CMMs, Vision Systems, and connected digital gages with 1factory's manufacturing quality control software. Improve speed and accuracy of inspection data collection. Supported devices include Zeiss, Keyence, PCDMIS, Mitutoyo and many more. Learn More
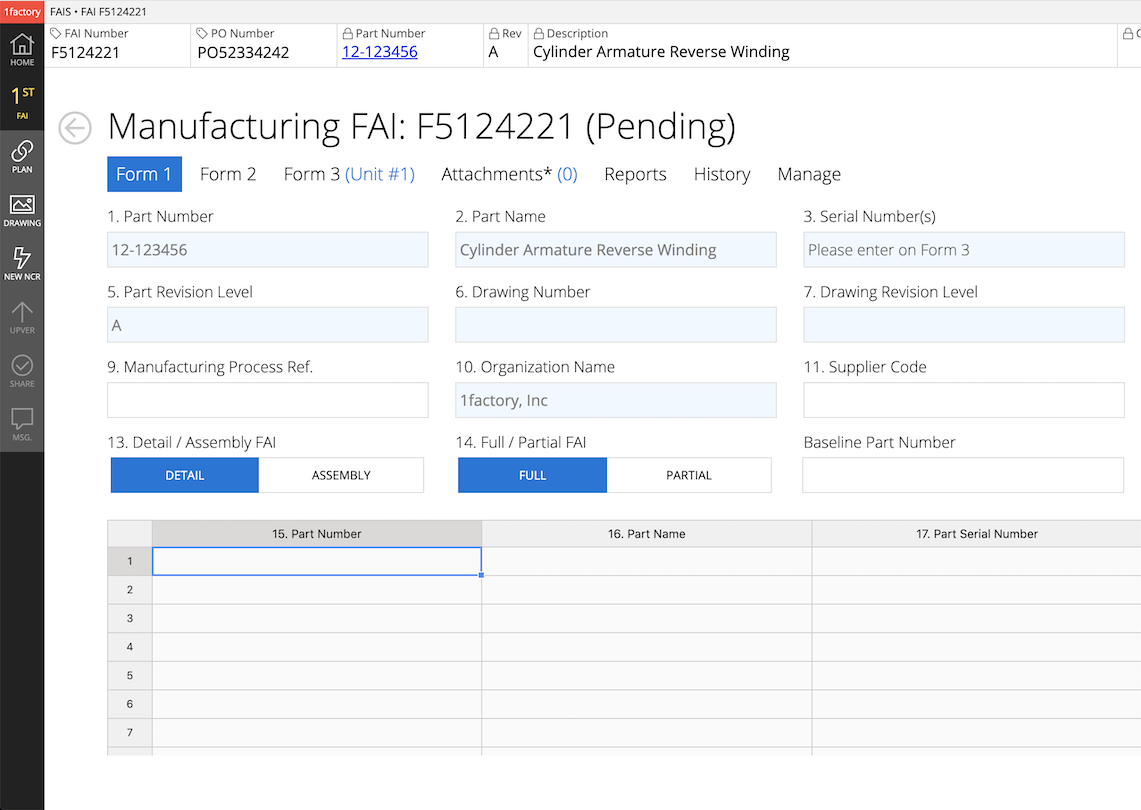
9In-Process Inspection
Ensure inspections are performed at each required step in the manufacturing process with 1factory's Inspection Management software. Enforce frequency based, AQL-based, or AS13002 (Ppk-based) sampling. Record hand-held tool and CMM data. Detect problems early in the manufacturing process, and reduce rework and scrap.
10Process Capability Cpk, Ppk
Enjoy automated Process Capability (Cp, Cpk, Pp, Ppk) calculations within 1factory's Inspection Management software. Supported features include: bilateral equal tolerances, bilateral unequal tolerances, unilateral tolerance, GD&T with bonus tolerances and more.
11Statistical Process Control (SPC)
Use individuals charts and Western Electric Rules to control manufacturing processes. Detect trends and anomalies quickly at the point of manufacture. Notify engineers and managers of out-of-control events.
12Final Inspection
Inspect finished parts and products with 1factory's Inspection Management software. Automate sample size calculation. Ensure all data is collected. Associate raw material and outside processing certs. Generate inspection reports for customers.
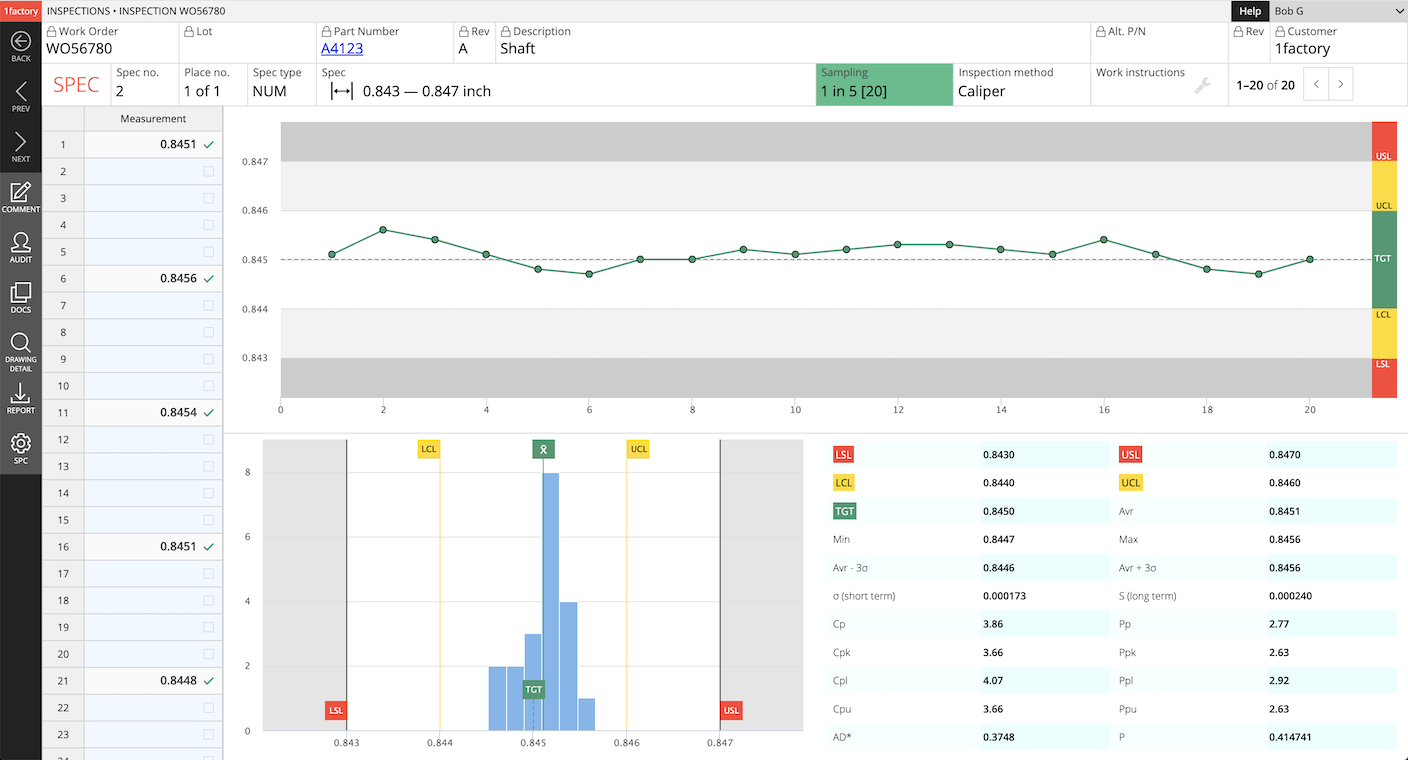
13PPAP Master Library
Build a reusable library of failure modes by Manufacturing Process (e.g. Mill-Turn, Grinding, Plating, Deburring etc.). Automatically populate a part-number-specific PFMEA with Failure Modes, Effects, Severity, Causes, etc. from the Library and save hours of time with 1factory's Manufacturing Quality Control software.
14PFMEA (AIAG, AS13100)
Build and maintain part-number-specific PFMEAs with version control. Identify product-feature and process failure modes. Output PFMEA reports in AIAG/APQP, or AS13000 formats with a single-click.
15Control Plan
Balloon your drawing, and extract features to a Control Plan with just a few clicks. Assign Inspection Methods, Sampling Rules, and Reaction Plans to a single parameter or a group of parameters. Output Control Plan in AIAG/APQP standard format with a single click.
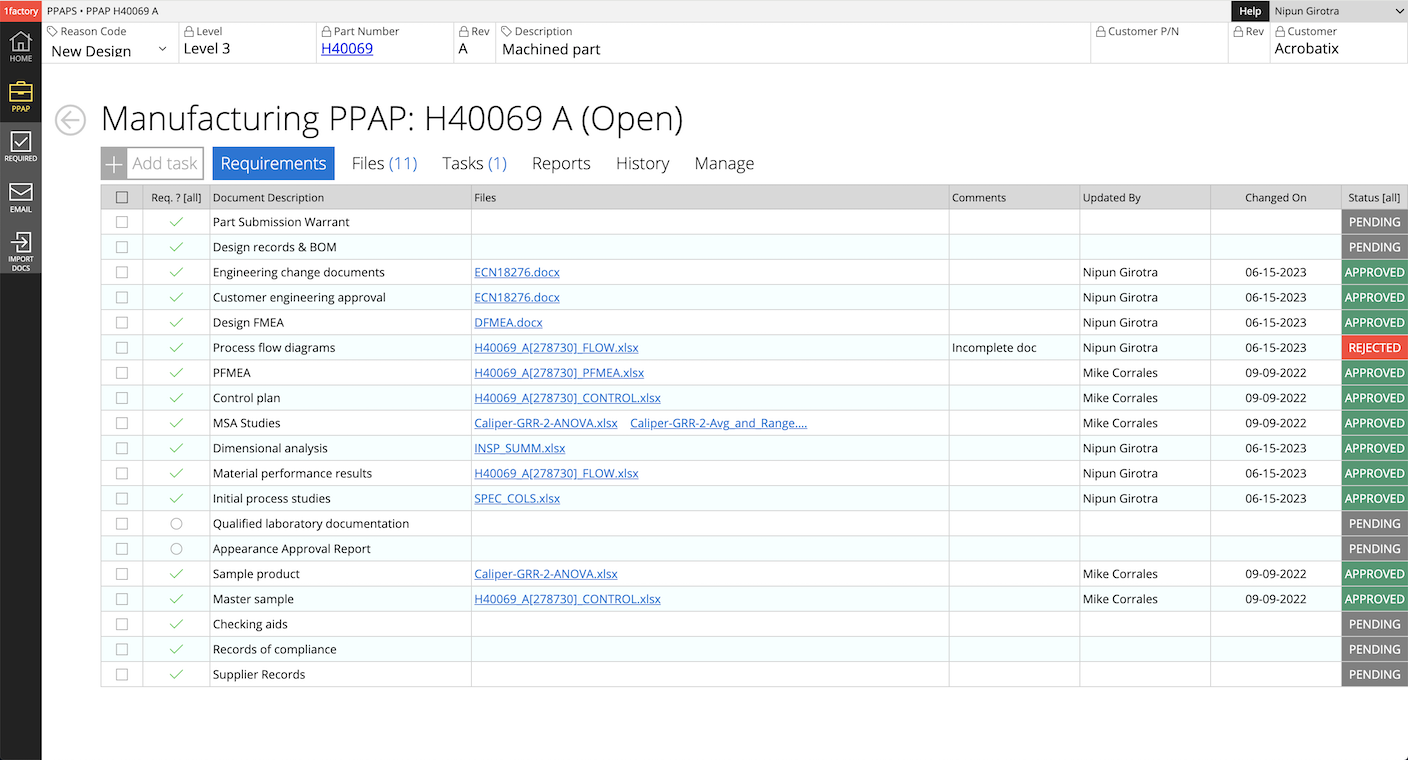
16PPAP Log
Assemble the various PPAP elements - Process Flow, Control Plan, PFMEA, Measurement Data, Certs, Gage R&R etc. - from engineering, manufacturing, quality control etc. into PPAP packages for customers. Create and manage delta PPAPs as processes change.
17Gage Calibration
Easily manage calibration records for thousands of gages with 1factory's Manufacturing Quality Control software. Log actual calibration data against gage tolerances and/or attach certs. Receive notifications when gages are due for calibration.
18Gage Issue / Return
Transact Gages between Tool Crib, Factory Floor, and Calibration Vendors with simple barcode scanning. Track gages by location, machine, work order, calibration vendor and user.
19Gage Kits
Organize and manage part-number-specific kits of gages e.g. pin gages, thread gages, or other gages that are custom to a part or a family of parts. Easily find and transact all the gages required for a given part.
20Gage R&R / MSA
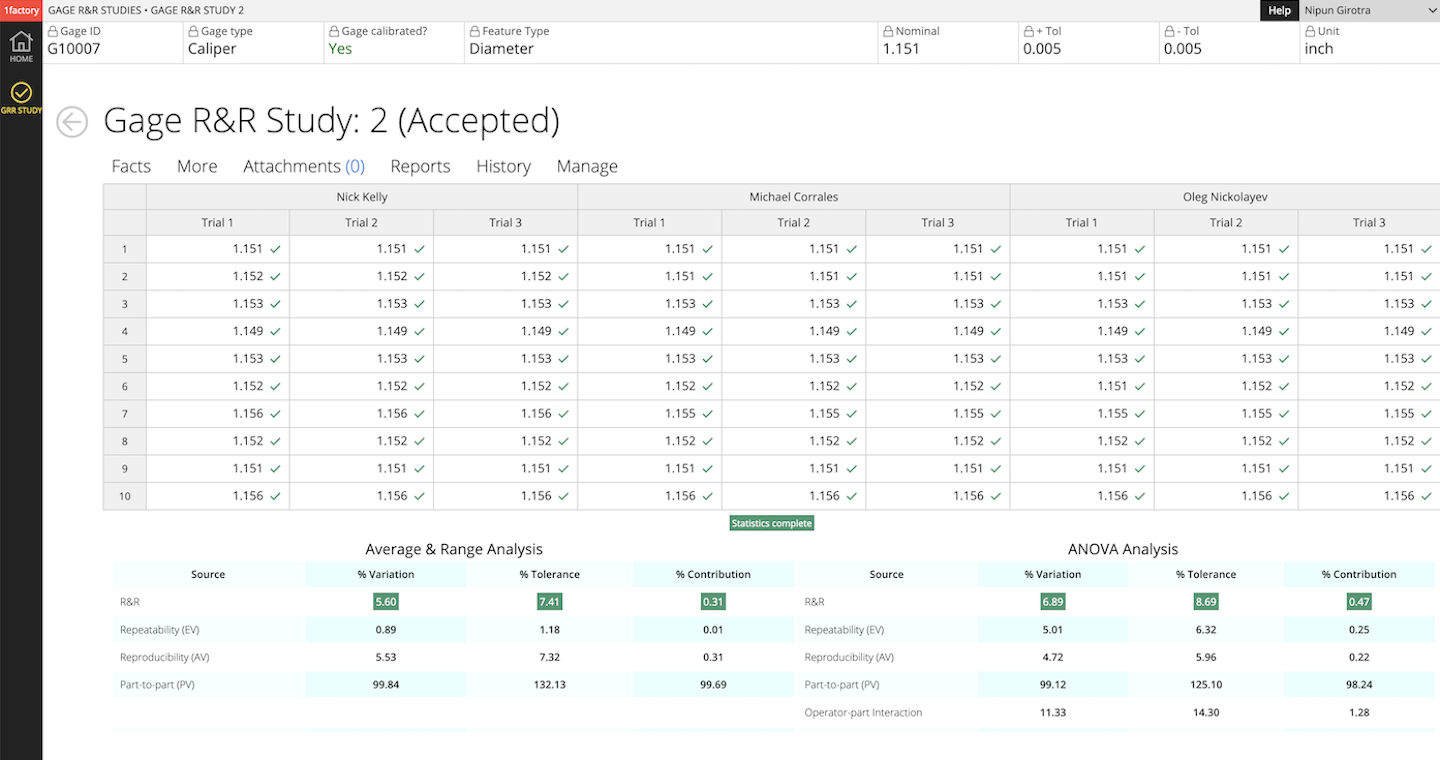
Perform Variable Gage R&Rs. Configure number of operators, parts and trials. Instantly generate reports for both the ANOVA and Range methods. Accept/reject based on NDC, % Tolerance, % Contribution etc.
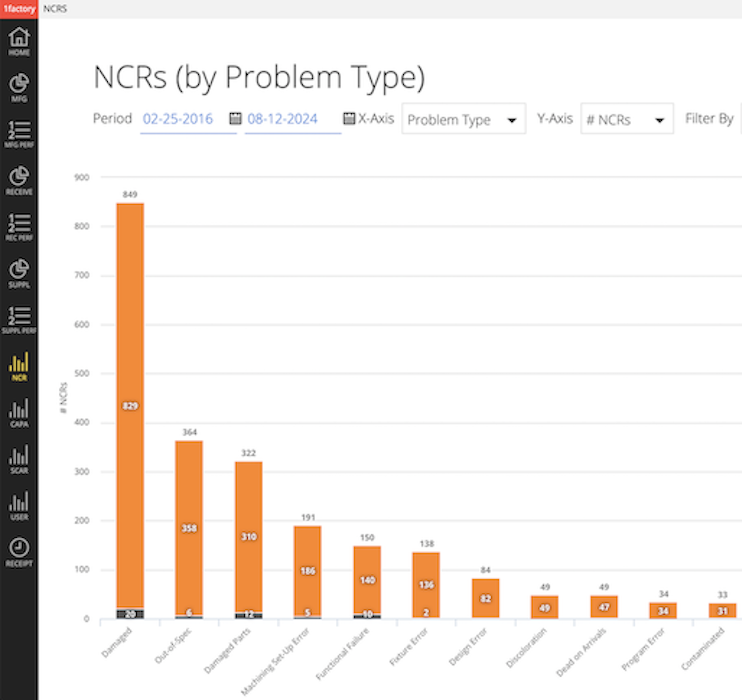
21NCRs, CAPAs, SCARs, Complaints
Record and track issues reported by incoming inspection, manufacturing, field service, and customers with 1factory's Issues management capability. Pareto and filter issues by problem type, root cause, date-range, part number, supplier, etc.
22Traceability
Track and retrieve manufacturing inspection data by Work Order, Serial Number, Date Code, Operator, Machine ID, Spindle, Cavity, Gage ID, Measurement Date & Time. Ensure uniqueness of serial numbers across inspection lots. Easily retrieve data for containment and recalls.
23Real Time Analytics
Instantly analyze measurement data with built-in lightning-fast analytics without expensive 3rd-party BI tools. Analyze data across work-orders and drive process improvements.
24Audit Readiness
Digitize all manufacturing quality control activities. Ensure gages are calibrated, measurements are recorded, and required certs are attached. Track non-conformances to completion. Always be ready for an audit.
25Calculated Specs
Automate the calculation of derived values such as averages, deviations, sums, differences, products, ratios etc. Save hours of calculation time and eliminate errors.
26Multi-Parameter SPC
Monitor all critical parameters with multiple run charts on a single screen. Equip operators with tools to detect and address process anomalies in real-time.
27Inspections by Operation
Automate the creation of inspection records for each Operation (and optionally for each machine). Set varying lot sizes for each Operation (and/or machine) to accomodate varying capacities and yield loss. Track completion status and yield by Operation.
28Validation & eSignatures (21 CFR Part 11)
Quickly validate the system with a validation package from 1factory. Capture user signatures every time a unit of work is completed. Learn More

CASE STUDIES
Read Highlights & Transcript
Highlights: Consolidated Quality Systems & CMM Automation
- Software Consolidation: UMC eliminated 5 separate Quality Control systems, unifying all operations into 1factory.
- CMM Auto-Upload: Automated data collection from 20 CMMs, processing over 1 million measurements monthly.
- Real-Time SPC: Replaced manual transcribing with instantaneous data refresh for immediate process corrections.
Transcript Excerpt: "The old systems were slow... often faster to do work manually. With 1factory, paper and Excel have been completely eliminated from our QC activities."
Ultra Machining Company
Ultra Machining Company (UMC) is a manufacturer of high-precision components for medical device, aerospace, and defense applications. The products are complex, with 100 - 800 features per product.
With 1factory's manufacturing quality control software, UMC successfully consolidated 5 separate QC systems into 1.
Today, data is automatically uploaded from 20 CMMs and 8 VCMMs, 24 hours a day. Over 1 million measurements are recorded in 1factory each month. Auto-ballooning speeds up FAI creation. And hours are saved from each PPAP package creation.
Read the Modern Machine Shop Feature on 1factory's work with UMC.
Watch a 4-minute video to hear first-hand experiences from the cross-functional UMC team: Machinists, Quality Engineers, Director of Quality, VP of IT, and VP of Operations.
Read Highlights & Transcript
Highlights: Large-Scale Medical Device QC Deployment
- Massive Scalability: Deployed 1factory across 4 sites, 900+ machine tools, and 1,300 users within a single year.
- High-Volume Data: Successfully managing 21,000+ inspection plans and recording 12 million+ data points annually.
- Mission-Critical Quality: President John Braun states, "Quality is the number one thing we sell. We have to have the best quality systems in the world."
Transcript Excerpt: "We move from 6 software systems to a single all-in-one QC software system. 1factory has been a true partner in our growth."
rms - A Cretex Medical Company - Medical Devices
rms (Cretex), is a leader in medical device manufacturing for the Interventional, Cardiac Rhythm Management, and Orthopedic markets.
When rms set out to replace their legacy systems, they sought a partner capable of meeting their challenging requirements, along with ease of implementation, ease of use, and scalability.
In just one year, rms successfully deployed 1factory for: 4 manufacturing sites, 900+ machine-tools, 1300 users and 100+ CMM / vision systems.
rms created 21,000+ Inspection Plans, inspected over 70,000+ Lots, recorded 12 million+ Data Points and consolidated 6 QC systems into one.
Watch a 4-minute video to hear first-hand experiences from the cross-functional rms team: Machinists, Quality Engineers, Quality Managers, Project Manager and President.
Read Highlights & Transcript
Highlights: Medical Device Compliance & Strategic Advantage
- Validation Simplified: 1factory's validation testing and documentation eliminated massive burdens for FDA compliance.
- Strategic Support: CIO Robin H. highlights that 1factory stayed as a partner "every step of the way" after the contract was signed.
- Data Accessibility: Leveraging automated CMM data collection to ensure the right data is available at the right point.
Transcript Excerpt: "With 1factory, they really do such a phenomenal job at their validation documentation. It helps us gain a competitive edge when customers visit."
Avalign Technologies - Medical Devices
"There are thousands of software suppliers to select from. And during the sales cycle you often find that the relationship is terrific, but then it falls apart very quickly after you sign the contract. With 1factory, they stayed with us every step of the way, and they continue to partner with us into our future." Robin H., CIO
Avalign Technologies is an industry leader in the design and manufacturing of high-precision implants and instruments for orthopedic, spine, and trauma applications.
The Challenge: Avalign needed a comprehensive Quality Control solution that delivered: Robust capabilities, Enterprise-grade security, Seamless scalability, Intuitive user experience, and Initial and on-going validation support.
Watch a 4-minute video to hear first-hand from the Avalign team about their experience with 1factory.
Read Highlights & Transcript
Highlights: Aerospace AS9102 Success & Instant Go-Live
- Unmatched Implementation: Replaced a projected 9-month competitor installation with a live, 1-day deployment.
- AS9102 Success: Completed over 500 First Article reports, cutting FAIR time from hours to minutes.
- "Just Like the Demo": CEO Rodney B. confirms 1factory worked exactly as promised from day one.
Transcript Excerpt: "Within twenty minutes, I had bubble-printed the plans and inspection sheets. It eliminated paperwork errors for our most critical parts."
Next Intent - Aerospace, Space, Defense
Next Intent is a world-class precision manufacturer, serving the space, defense, aerospace and semiconductor industries. The parts they craft are critical to the success of billion-dollar, multi-decade missions — where a single failure isn't just costly, it's catastrophic.
Notable projects include components for THREE Mars missions, and the revolutionary Red Camera Assembly for the Giant Magellan Telescope and more.
Next Intent picked 1factory to meet its challenging quality requirements. With 1factory, Next intent has unified quality planning, inspections, gage managements, and quality records into one seamless ecosystem, automating paperwork, eliminating errors, and helping deliver the highest quality parts to customers.
"The Software worked just like the demo!" Rodney B. President & CEO.
"We were going to go with another company's software. The implementation time was nine months. That was too long. With 1factory, they were getting it to work within the first day."
EASY TO USE. LIGHTNING FAST. SECURE & RELIABLE
INTUITIVE DESIGN & LIGHTNING FAST SPEED
1Factory's intuitive interface and streamlined workflows make training effortless - most teams achieve full deployment in under two hours with zero operational disruption.
Speed is non-negotiable on the shop floor. Every interaction - from data entry to ballooning to search - happens instantaneously. Your manufacturing operations run at full pace, never waiting on software. With 24/7/365 global availability and real-time analytics, 1Factory keeps your quality operations moving at the speed of production.
SECURITY & RELIABILITY
1Factory meets the security standards that matter to regulated manufacturers: ITAR-compliant hosting on AWS GovCloud, full NIST 800-171 compliance, and SOC 2 Type 2 certification. Your quality data stays protected and audit-ready.
Over the past decade, 1Factory has maintained >99.99% uptime - because we understand that your quality system can't afford downtime. Data encryption, daily database snapshots, and redundant file storage ensure your critical quality records are always protected and recoverable.
FAQ: MANUFACTURING QUALITY CONTROL
What are some of the challenges manufacturers face in quality control? What are the consequences?
Manufacturers face increasing product, process, and documentation complexity, making it difficult to ensure product quality and meet compliance requirements. As a consequence, manufacturers struggle with poor yield and low productivity. Challenges include:
Quality planning remains a slow manual process. Manual drawing ballooning and control plan creation processes consume excessive engineering time.
PPAP documentation is a significant burden. Creating Process Flow, PFMEA, and Control Plans requires substantial time and resources.
Change control is especially challenging. With an ever-increasing number of parts to manufacture, product features to manage, and frequent design changes, it becomes nearly impossible to ensure every team member at every operation is working with the latest revision of each document.
Data collection and reporting is typically manual. Data must be transcribed from hand-held gages, CMMs, and Vision Systems.
Process capability analysis is difficult to perform. Data must be transferred from paper or stand-alone systems before analysis can begin.
Process control difficulties are widespread. These include managing inspection requirements across multiple operations and machine setups, and collecting and consolidating data from diverse inspection equipment and formats.
Gage calibration and traceability across systems is challenging. Maintaining calibration schedules and ensuring gage traceability becomes complex across multiple platforms.
Reporting burdens are extensive. These include managing part families and tabulated drawings with multiple configurations, maintaining traceability of measurements to specific lots, operators, and equipment, and validating inspection processes for regulatory compliance.
The Impact
Without proper tools, these challenges lead to delayed product launches, inconsistent documentation, and quality issues during production. Quality teams spend excessive time on manual documentation rather than process improvement, while engineers struggle to maintain consistency across multiple product launches and ongoing production requirements.
How does 1factory address manufacturing quality control challenges? What is the Return on Investment?
1factory dramatically simplifies and automates every step of the manufacturing quality control process, helping manufacturers deliver world-class quality and meet compliance requirements.
Quality Planning: 1factory streamlines the creation of control plans, cutting time from hours to minutes through automated ballooning, revision comparison, and more.
PPAP Management: 1factory simplifies PPAP creation process, cutting PPAP preparation time from days and weeks to hours, with a reusable library of failure modes and control plan elements.
Change Control: 1factory makes it easy to manage quality for thousands of part numbers, each with hundreds of characteristics (features) that must be controlled. 1factory also make it easy to manage drawing revision changes as well as changes to sampling rules, inspection methods and ops sheets.
Data Collection and Reporting: The 1factory platform revolutionizes data collection from CMMs, vision systems, and digital gages, providing operators with real-time feedback and automated alerts.
Process Capability Analysis 1factory automates process capability calculation savings hours of analysis time.
Statistical Process Control: Built-in statistical process control (SPC) and comprehensive process capability analysis enable immediate detection of process shifts, giving operators and engineers a complete view of process performance.
Gage Calibration: 1factory's deeply integrated gage calibration module ensures gages are always calibrated and helps ensure gage traceability across inspection records.
Reporting: Reporting becomes effortless with automated generation of First Article Inspection reports, PPAP documentation, and industry or customer-specific report packages. Gage management is transformed through automated calibration tracking, comprehensive Gage R&R studies, and efficient tool crib management.
Root-Cause Analysis: Issues are tracked from initial detection through root cause analysis and corrective action. Real-time analytics help identify trends and systemic issues, enabling continuous quality improvement.
Return on Investment:
By replacing disconnected systems and manual processes with 1factory's all-in-one Manufacturing Quality Control Software, manufacturers can speed-up quality control tasks, improve productivity, improve factory yield, and deliver world-class quality.
500+ CUSTOMERS. 30+ COUNTRIES. 20+ INDUSTRIES.

AEROSPACE & INDUSTRIAL

AEROSPACE MACHINING

PRECISION MACHINING

MEDICAL DEVICES

AUTONOMOUS AIRCRAFT

ADVANCED CERAMICS