Complete Guide to Corrective and Preventive Action (CAPA)
Table of Contents
- 1. Introduction: The Power of CAPA
- 2. What is CAPA? And When is it Needed?
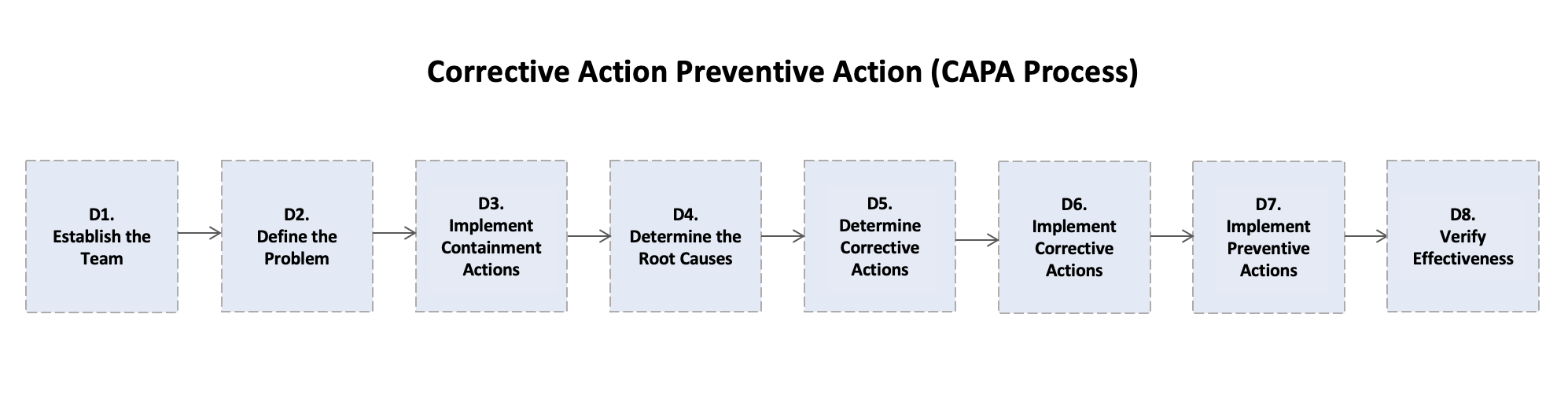
- 3. The 8D Process for CAPA: A Structured Approach
- D1: Establish the Team
- D2: Describe the Problem
- D3: Implement Interim Containment Actions
- D4: Determine Root Causes
- D5: Develop Permanent Corrective Actions (PCAs)
- D6: Implement & Validate Permanent Corrective Actions
- D7: Implement Preventative Actions
- D8: Verify Effectiveness & Close the 8D
- 4. Case Study: Rusting Valve Bodies - An 8D Problem-Solving Approach
- 5. The Benefits of Digital CAPA Management
1. Introduction: The Power of CAPA
The Corrective Action Preventive Action (CAPA) process focuses on solving significant or recurring problems (Corrective Action), and preventing the recurrence of the same or similar issues (Preventive Action). When a problem warrants a deeper investigation, the CAPA process, often guided by the 8 Disciplines (8D) problem-solving methodology, ensures thorough analysis and effective solutions. This leads to high product quality, improved regulatory compliance, supports a culture of continuous improvement, and ultimately leads to improved customer satisfaction.
A CAPA helps organizations learn from quality challenges; instead of just reacting to individual issues, it provides a structured way to identify systemic weaknesses and drive lasting improvements.
2. What is CAPA? And When is it Needed?
A nonconformance is any failure to meet required standards, from raw materials to customer delivery. While many nonconformances can be resolved through immediate rework or simple adjustments, a CAPA is typically initiated for nonconformances that:
- Are significant: Major quality failures affecting product safety, performance, or customer satisfaction.
- Are recurring: Minor issues that keep happening, indicating a systemic problem.
- Pose a high risk: Potential problems identified through risk assessments, audits, or trend analysis that could have a significant negative impact if they occur.
- Are required by regulation or customer mandate: Specific issues that necessitate a formal CAPA investigation.
Corrective Action: These are steps taken to eliminate the root causes of an existing nonconformance, ensuring it doesn't return.
Preventive Action: These are steps taken to stop potential problems from happening in the first place, proactively managing identified risks or applying lessons learned from past issues to prevent future ones.
Combined, CAPA is a powerful tool for continuous improvement and risk management, transforming quality events into opportunities for a stronger system.
3. The 8D Process for CAPA: A Structured Approach
The 8D (Eight Disciplines) problem-solving method provides a clear roadmap to systematically find, fix, and eliminate recurring problems.
D1: Establish the Team
What we're doing: Assembling a group with complete, relevant experience and skills.
Why: To ensure all angles that could be causing or are impacted by the issue are covered.
D2: Describe the Problem
What we're doing: Defining the issue clearly and measurably.
Why: To ensure focus on the actual problem, preventing wasted effort on symptoms.
D3: Implement Interim Containment Actions
What we're doing: Taking immediate, temporary steps to protect the customer and stop the problem from worsening.
Why: To limit damage and provide time for thorough investigation and permanent solutions.
D4: Determine Root Causes
What we're doing: Uncovering all underlying reasons for the problem.
Why: To ensure permanent fixes target the true source, preventing recurrence.
D5: Develop Permanent Corrective Actions (PCAs)
What we're doing: Creating and selecting the best long-term solutions.
Why: To effectively eliminate root causes and ensure the problem doesn't return.
D6: Implement & Validate Permanent Corrective Actions
What we're doing: Putting chosen solutions into practice and confirming their effectiveness.
Why: To ensure corrective actions work in the real world and can be maintained.
D7: Implement Preventative Actions
What we're doing: Changing systems and procedures to stop similar problems in the future.
Why: To institutionalize learning, prevent recurrence across the organization, and strengthen the QMS.
D8: Verify Effectiveness & Close the 8D
What we're doing: Confirming all actions worked and formally closing the process.
Why: To ensure the problem is truly solved, foster continuous improvement, and provide closure.
4. Case Study: Rusting Valve Bodies - An 8D Problem-Solving Approach
Problem Statement: A customer reported receiving a batch of machined oil and gas valve bodies with visible surface rust, rendering them unusable for immediate assembly and risking critical delays in their project. This is a critical quality escape as the parts should have arrived in a corrosion-free state, protected for transit and storage.
D0: Plan & Prepare
Objective: To investigate the root cause of the rust on valve bodies and implement effective corrective and preventive actions to prevent recurrence.
Initial Assessment: Customer complaint received, affected batch identified (Batch #VG-2025-07-001, 150 units).
Resources: Quality Incident Report (QIR-2025-07-005) initiated.
D1: Establish the Team
A cross-functional team was assembled with expertise relevant to the product and process:
- Team Lead: Quality Manager
- Members:
- Manufacturing Engineer (Machining)
- Process Engineer (Cleaning & Preservation)
- Logistics/Packaging Specialist
- Supplier Quality Engineer (for raw material or subcontracted processes if applicable)
- Customer Service Representative (for customer liaison)
D2: Describe the Problem
- What: Machined oil and gas valve bodies exhibiting reddish-brown surface rust.
- Where: Rust observed on external machined surfaces, particularly in recessed areas and threads. Found upon receipt at the customer's facility and confirmed by internal inspection of retained samples from the same batch.
- When: Discovered on July 25, 2025, from a shipment dispatched on July 15, 2025.
- Who: Customer's receiving inspection department reported the issue.
- How: Visual inspection revealed rust.
- How Many: 100% of the received batch (150 units) were affected. An additional 50 units from the same production lot in internal stock were inspected, and 80% (40 units) also showed signs of rust.
- Severity: High impact - customer production halted, potential for reputation damage and significant financial loss due to scrap and rework.
D3: Implement Interim Containment Actions
Immediate actions to protect the customer and prevent further spread:
- Customer Communication: Informed the customer of immediate actions being taken and arranged for immediate return of affected parts.
- Product Segregation: All remaining units from Batch #VG-2025-07-001 in internal stock were immediately quarantined and physically segregated in a dedicated "HOLD" area.
- Incoming Inspection Hold: A temporary hold was placed on all finished valve bodies awaiting shipment until further investigation and verification of the rust prevention process.
- 100% Inspection & Rework/Scrap:
- Customer-returned parts were 100% inspected. All rusted parts were deemed non-conforming. Options considered: chemical rust removal followed by re-preservation (if feasible and validated) or scrap. Due to the nature of oil and gas components, scrap was chosen for critical parts to ensure integrity.
- Internal quarantined stock was also 100% inspected. Rusted parts were dispositioned as scrap.
- Enhanced Preservation for New Shipments: For critical urgent orders, an enhanced temporary rust prevention application (e.g., thicker layer of volatile corrosion inhibitor (VCI) oil, additional VCI packaging) was implemented with documented verification before shipment.
D4: Determine Root Causes (Root Cause Analysis - RCA)
The team used the "5 Whys" and Ishikawa (Fishbone) Diagram to identify potential causes across Manpower, Machine, Material, Method, Measurement, and Environment:
Initial Hypotheses:
- Inadequate rust preventative application.
- Contamination during manufacturing or handling.
- Improper packaging.
- Environmental factors during storage or transit.
Investigation and Findings:
- Why did the parts rust? Because the protective film on the machined surfaces was compromised or insufficient.
- Why was the protective film compromised/insufficient?
- Method (Primary Cause): The existing post-machining cleaning process (alkaline wash) was effective at removing cutting fluids, but the subsequent drying process was found to be inconsistent. Residual moisture was trapped in threads and recessed areas, creating an environment for flash rust. The application of the rust preventative oil was also found to be manual and not consistently covering all surfaces, especially internal geometries.
- Material (Contributing Cause): Analysis of the rust preventative oil showed it met specifications, but its effectiveness was highly dependent on a completely dry surface and thorough application. The current concentration and type of rust preventative might be insufficient for prolonged exposure to humidity fluctuations.
- Environment (Contributing Cause): Inspection of the packaging revealed that while VCI paper was used, it wasn't consistently sealing the parts from external humidity, particularly during transit through varying climate zones. Warehouse humidity control was also found to be marginally effective in certain areas.
- Measurement (Escape Cause): The final inspection for rust was visual only and performed in an area with good lighting, but without specific humidity or residual moisture checks. The standard transit preservation method was deemed insufficient for the actual transit conditions experienced.
Verified Root Causes:
- Inconsistent Drying Process: Residual moisture remained on machined surfaces, particularly in blind holes and threads, after the wash cycle due to insufficient air-knife drying time and pressure settings for complex geometries.
- Sub-optimal Rust Preventative Application Method: The manual application of rust preventative oil did not ensure complete and uniform coverage, especially in hard-to-reach internal features of the valve bodies.
- Inadequate Packaging for Environmental Conditions: Standard packaging (stretch wrap and VCI paper) did not provide sufficient long-term humidity protection for extended transit times or varying climatic conditions, allowing moisture ingress and condensation.
D5: Develop Permanent Corrective Actions (PCAs)
Solutions directly addressing the identified root causes:
- Automate Drying Process & Optimize Parameters:
- Install an automated air-knife drying station with programmable logic control (PLC) to ensure consistent drying cycles based on part geometry.
- Conduct trials to optimize air pressure, temperature, and drying time to ensure 100% moisture removal from all surfaces, including internal features.
- Implement Automated Rust Preventative Application:
- Investigate and procure an automated dipping or spray system for rust preventative oil application to ensure uniform and complete coverage of all surfaces.
- Alternatively, for immediate implementation, revise work instructions to include a mandatory visual check of oil coverage using a UV lamp (if oil is UV traceable) for manual application, and increase oil viscosity or add a more robust VCI additive.
- Upgrade Packaging & Storage Conditions:
- Switch to heat-sealable VCI bags or sealed anti-corrosion barrier bags for individual valve bodies or smaller batches.
- Utilize desiccant packs within the sealed packaging to absorb any residual moisture or moisture ingress during transit.
- Implement humidity monitoring and control in the finished goods warehouse, particularly for long-term storage areas.
D6: Implement & Validate Permanent Corrective Actions
Implementation Plan:
- Phase 1 (Immediate - Automated Application & Packaging):
- Automated rust preventative system/revised manual application: By August 15, 2025.
- New packaging materials & procedures: By August 20, 2025.
- Warehouse humidity control: By September 30, 2025.
- Phase 2 (Long-Term - Automated Drying):
- Automated drying station procurement/installation: By October 31, 2025.
Validation Activities:
- Salt Spray Testing: Conduct salt spray tests (ASTM B117) on sample parts processed with the new drying and preservation methods. Target: No rust after 96 hours.
- Humidity Chamber Testing: Expose treated parts to high humidity (e.g., 90% RH, 40°C) for extended periods (e.g., 2 weeks) to simulate aggressive transit/storage conditions. Target: No rust.
- Shipping Trials: Monitor trial shipments to customer locations with varying climates, including opening and inspecting parts upon arrival.
- First Article Inspection (FAI): Conduct FAI on parts produced with the new processes, including visual inspection and residual moisture checks.
- Standard Operating Procedure (SOP) Updates: Revise all relevant SOPs for cleaning, preservation, packaging, and inspection.
- Training: Train all affected personnel on new procedures.
D7: Prevent Recurrence
Actions to institutionalize the changes and prevent similar issues across other products/processes:
- Process Control Plan Update: Incorporate new process parameters (e.g., air-knife settings, automated spray/dip parameters, packaging specifications) into the control plan.
- Quality Control Checkpoints: Add mandatory checks for residual moisture (e.g., litmus paper, moisture meter) post-drying and visual inspection of rust preventative coverage to the final inspection.
- Supplier Management: Review rust prevention and packaging requirements with raw material suppliers or sub-contractors, especially for pre-machined components.
- Design Review Integration: Incorporate "Design for Manufacturability and Preservation" principles into new product development, considering complex geometries and their impact on cleaning/drying/preservation.
- Preventive Maintenance: Schedule regular maintenance and calibration for drying equipment and rust preventative application systems.
- Training Matrix Update: Ensure all operators and quality inspectors are trained and certified on the updated procedures.
- FMEA Review: Update Process FMEA (PFMEA) to reflect new failure modes related to rust prevention and assign appropriate controls and detection methods.
D8: Congratulate the Team & Close the 8D
- Team Recognition: The team's efforts in resolving a critical customer issue were formally recognized and celebrated.
- Knowledge Transfer: The lessons learned from this 8D were documented and shared across relevant departments to foster continuous improvement.
- Formal Closure: The 8D report (QIR-2025-07-005) was formally closed after verification of effectiveness and successful implementation of preventive actions. The customer was notified of the completed actions and long-term improvements.
Verification of Effectiveness (Ongoing Monitoring):
- Reduced Customer Complaints: Monitored customer complaints for 6 months post-implementation; zero rust-related complaints were received for valve bodies.
- Internal Quality Audits: Regular internal audits confirmed adherence to new drying, preservation, and packaging SOPs.
- Process Performance Data: Collected data on critical process parameters (e.g., air-knife pressure, humidity levels) showing consistent performance within specified limits.
- Long-Term Storage Samples: Samples stored internally under typical warehouse conditions remained rust-free after 3 months, 6 months, and 1 year.
- Cost Savings: Reduction in scrap/rework costs associated with rust and improved customer satisfaction.
5. The Benefits of Digital CAPA Management
While manual CAPA is possible, digital systems streamline the process, offering significant advantages:
- Streamlined Workflows & Better Teamwork: Real-time visibility and automated tasks improve collaboration and speed up the CAPA process.
- Deep Root Cause Analysis & Prevention: Structured templates and access to historical data reveal patterns, ensuring thorough analysis and preventing recurrence.
- Tracking Quality Costs & Smarter Decisions: Automatic cost calculation helps prioritize improvement efforts based on financial impact.
- Compliance with Full Audit Trails: Tamper-proof records simplify regulatory adherence and prepare organizations for audits.
Modern digital systems, such as 1factory's Quality Management Software transform problem-solving from a burden into a strategic advantage, driving continuous improvement and a stronger competitive edge.